Cancuravax is an Oncolysate cancer vaccine for the treatment of Advanced Melanoma, with NO TOXICITY AND A PROVEN HIGH RATE OF REGRESSION (CURE). It offers incredibly long-term survival for many patients - unparalleled against virtually all approved agents.
Cancuravax Pty Ltd is an Australian biotechnology company established in 2019 to develop, and take to market, this unique therapeutic vaccine (Cancuravax). Cancuravax holds exclusive licence to broadranging patents.
The Clinical Trials, reported in 2010 and 2014, used the vaccine in advanced melanoma (very ill patients), showing HIGH COMPLETE RESPONSE (CR) RATES WHERE ALL MELANOMA HAS DISAPPEARED, WITH LONG-SURVIVAL > 10-YEARS IN SOME PATIENTS AND THE LONGEST SURVIVOR, WITH VACCINE TREATMENT ALONE, IS 21+ YEARS.
Not only does Cancuravax have proven efficacy in ADVANCED STAGE 4 MELANOMA, but it also has significant potential to successfully treat OTHER CANCERS.

Melanoma of the skin is a common cancer in many countries. In about 10% of cases it spreads beyond the skin away from the site that it originated (metastatic) and involves the major body organs. Over the last few years immunotherapy, in general, has been shown to be mildly effective for treating metastatic melanoma and a number of drugs have been approved for clinical use. However, about 70-80% of patients fail these current therapies, and have continued progression to still die of their melanoma. This is a major area of unmet need where treatment is urgently required.
Our Onco-viral Lysate approach in Cancuravax offers a novel off-the-shelf agent for melanoma therapy – with a methodology possibly applicable to other cancer types.
Cancuravax has no toxic side-effects – none observed to date. In particular, no auto-immune side effects, or drug induced toxicity, or deaths were observed.
Cancuravax (Vaccinia Melanoma Cell Lysate (VMCL-vax)) vaccine has been shown to be very effective in treating advanced stage IV metastatic melanoma, demonstrating a high rate complete regression of melanoma (17%), and long-term survival of over 10-years and now over 21+ years currently in some patients.
Clinically useful responses occurred in 78% of treated patients. No, or minimal, side-effects were observed. Cancuravax offers an effective option in this space. Current approved drug therapies have serious side-effects which can limit therapy, and even cause death from the drug(s). They are costly and many are intravenous day-hospital treatments.
Cancuravax therapy is delivered easily by fortnightly injection into the skin in an outpatient setting. The potential advantages and application of Cancuravax is its simplicity with the lack of toxicity, ease of treatment, and high response rates in advanced melanoma management. This can be for patients that have failed current standard therapies (about 70- 80%, patients where essentially no effective treatment exists); or combined with newer therapies (inhibitor and immunotherapies). The clinical trial results experienced were unexpected and hugely significant.
GlobalData Melanoma – Epidemiology Forecast to 2026 Report Oct 2017. https://www.melanoma.org.au/understanding-melanoma/melanoma-facts-and statistics/?linkServID=A6B0737C-E570-AD05-C4E65883BAA177E2. Accessed 14 January 2018. Australian Institute of Health and Welfare 2017. Cancer in Australia 2017. Cancer series no. 101. Cat. no. CAN 100. Canberra: AIHW.
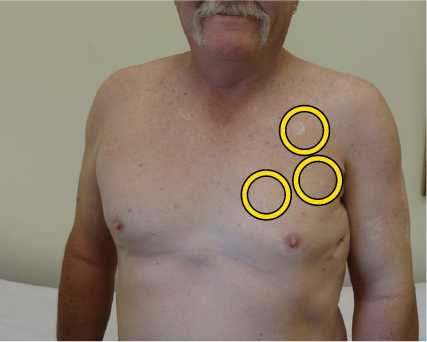
Yellow circles indicate locations of palpable lumps, which subsequently disappeared after vaccine treatment
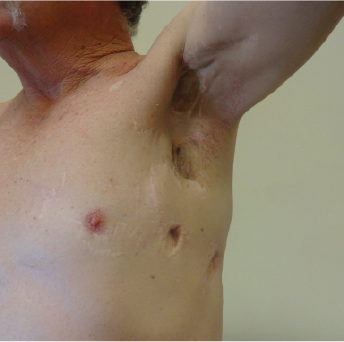
As can be seen, the Vaccine (alone) induced regression of established metastatic tumour (Nov 2000 to Dec 2000)
The patient had a Complete Response; Current Survival >19-years)
This patient had undergone multiple surgical resections of metastases but they had failed; and developed Stage IV melanoma. He had virtually no further treatment options and he asked to be treated with Cancuravax which had shown promise and no toxicity in earlier-Stage melanoma human clinical trials.
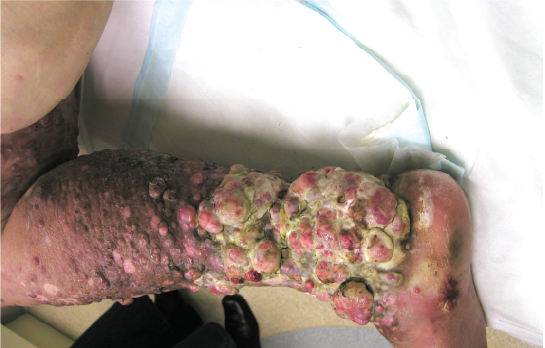
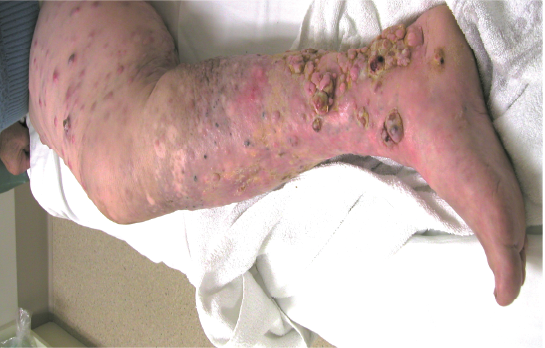
This woman had extensive bulky malignant melanoma metastases of the right leg. After treatment over a 4 month period, she had an almost complete regression of tumours. She lost kilograms of tumour from her leg using Cancuravax Vaccine Therapy alone, and could walk again.
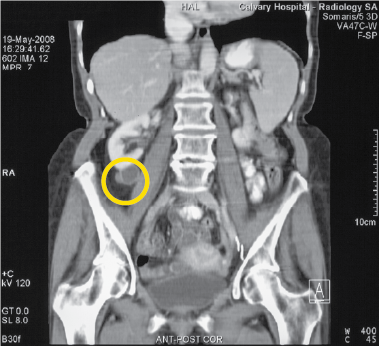
Yellow circle highlights Tumour
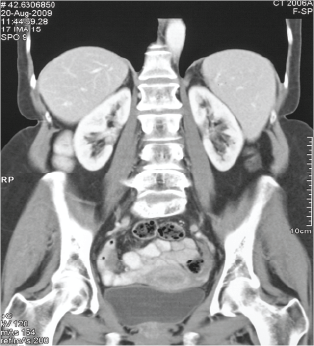
A 2nd round of Vaccine alone induced a complete regression of established metastatic Melanoma
(May 2008 to August 2009)
Recurrent cancer after CR
Current survival
18+ years (2019)
This woman had an initial complete response from Cancuravax melanoma vaccine therapy with added Dacarbazine chemotherapy in 2002, then all treatment was ceased. Metastatic melanoma developed below the right kidney in 2008, and Cancuravax was again given (alone) with complete regression of all melanoma to date (2020).

CancuraVax Oncolysate Immunotherapy is a “Phase 2/3” Oncolysate vaccine that represents the only (or one of the only) agents that has been clinically proven to produce a long-term (21-year so far) survival cure of advanced melanoma, with no toxic side effects.
Cancuravax is targeted at the area of major existing unmet need, where:
It is rare to get a clinically proven therapy/product at such an advanced state of development, ready for market.
Cancuravax is now actively seeking expressions of interest from companies to assist via partnership and/or to gain funding to assist further clinical trials, research and to gain appropriate Government approvals.
Our prime objective is for this treatment to reach the market as soon as possible.
CEO and Co-Founder
Andrew Lathlean
CSO and Co-Founder
Brendon Coventry
CFO and Co-Founder
Peter Hunt
CancuraVax Pty Ltd
c/- BDO Advisory (SA) Pty Ltd,
7th Floor, 420 King William Street,
Adelaide, SA, 5000
